Mole measurement, a cornerstone of chemistry, empowers us to quantify the unseen world of atoms and molecules, enabling precise understanding of chemical reactions, gas behavior, and solution concentrations. This exploration delves into the intricacies of mole measurement, unraveling its fundamental concepts, applications, and advanced techniques.
From the stoichiometry of chemical reactions to the behavior of gases under varying conditions, mole measurement provides a universal language for understanding the quantitative relationships between substances. It unlocks the secrets of solution chemistry, allowing us to tailor solutions with specific concentrations for diverse applications.
Mole Measurement: A Foundation for Chemical Understanding

Mole measurement is a fundamental concept in chemistry that allows scientists to quantify the amount of substance involved in reactions and other chemical processes. It provides a common unit of measure for comparing and manipulating different substances, ensuring accuracy and precision in scientific investigations.
Mole Measurement Fundamentals
A mole is defined as the amount of substance that contains exactly 6.02214076 × 10 23elementary entities, such as atoms, molecules, or ions. This number, known as Avogadro’s number, serves as a universal reference point for quantifying the number of particles in a given sample.
Mole measurement finds applications in various fields, including chemistry, physics, biology, and medicine. For example, in chemistry, it helps determine the molar mass of a compound, calculate the concentration of solutions, and balance chemical equations.
The number of moles in a sample can be calculated using the formula:
Number of moles = Mass of sample (in grams) / Molar mass (in grams per mole)
Mole Measurement in Chemistry
In chemistry, moles play a crucial role in understanding and manipulating chemical reactions. The mole concept enables chemists to determine the stoichiometry of reactions, predict the products and their quantities, and calculate the limiting reactant.
Moles are also essential for converting between mass and moles using molar mass. Molar mass is the mass of one mole of a substance and is calculated by adding the atomic masses of all atoms in the molecule or compound.
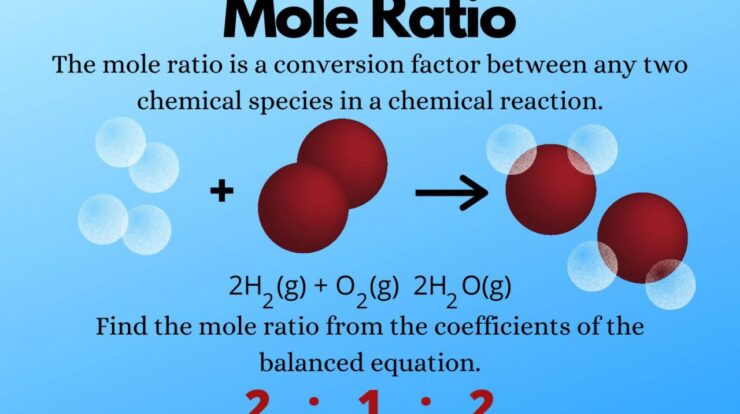
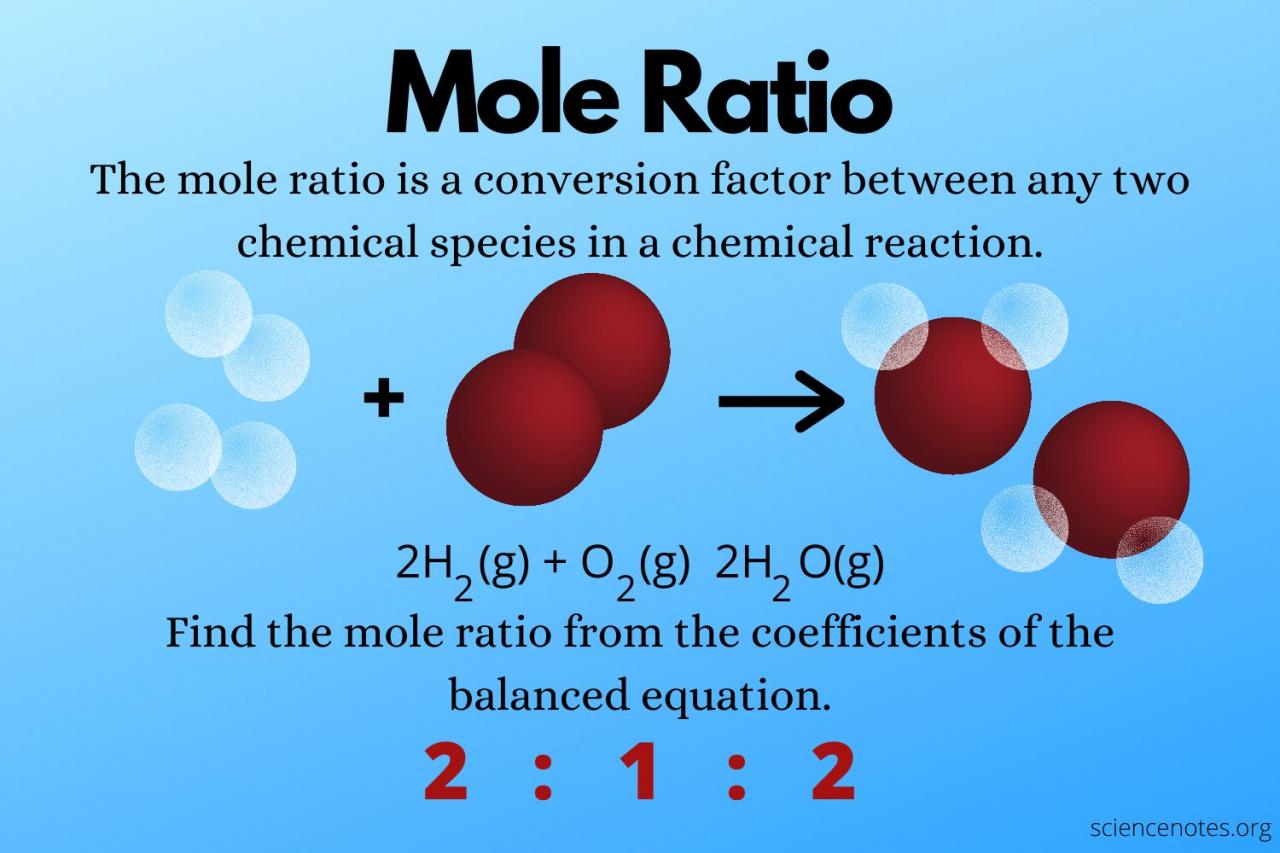
Stoichiometry involves using mole ratios to balance chemical equations and determine the amount of reactants and products involved in a reaction. For example, in the reaction between hydrogen and oxygen to form water:
H2+ O 2→ 2H 2O
The mole ratio of hydrogen to oxygen is 2:1, indicating that 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
Mole Measurement in Gas Laws
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas. It states that the product of pressure and volume is directly proportional to the number of moles and temperature.
Using the Ideal Gas Law, we can calculate the number of moles of a gas if its pressure, volume, and temperature are known. Additionally, moles can be used to determine the density and molar volume of a gas.
For example, if we have a container of gas with a volume of 22.4 liters at a temperature of 298 K and a pressure of 1 atm, the number of moles of gas can be calculated using the Ideal Gas Law:
n = PV / RT
where n is the number of moles, P is the pressure, V is the volume, R is the ideal gas constant, and T is the temperature.
Closure
Mole measurement extends beyond the confines of chemistry, finding practical applications in medicine, environmental science, and engineering. It underpins scientific research and technological advancements, enabling us to unravel the mysteries of the molecular world and harness its power for the betterment of society.
Question Bank
What is the significance of mole measurement in chemistry?
Mole measurement provides a standardized way to quantify the amount of substances involved in chemical reactions, ensuring accurate predictions of reaction outcomes and product yields.
How is mole measurement used in gas laws?
Mole measurement allows us to relate the volume, pressure, and temperature of gases through the Ideal Gas Law, enabling calculations of gas density and molar volume.
What is the practical application of mole measurement in medicine?
Mole measurement is crucial in determining drug dosages, ensuring accurate administration and optimizing treatment outcomes.